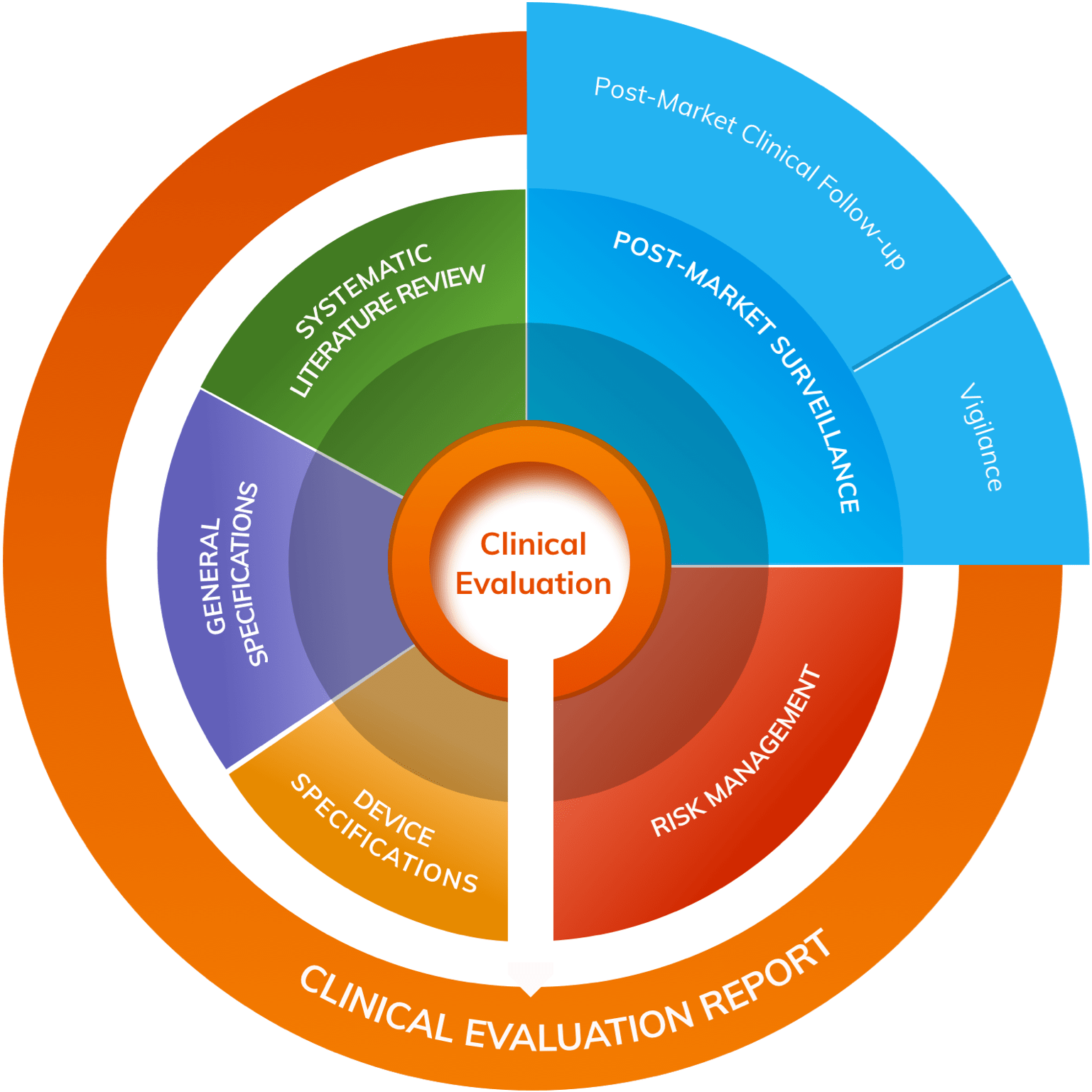
The Post-Market Clinical Follow-up (PMCF) requirements under the European Medical Device Regulation: Step by Step PMCF - GMED Medical Device Certification

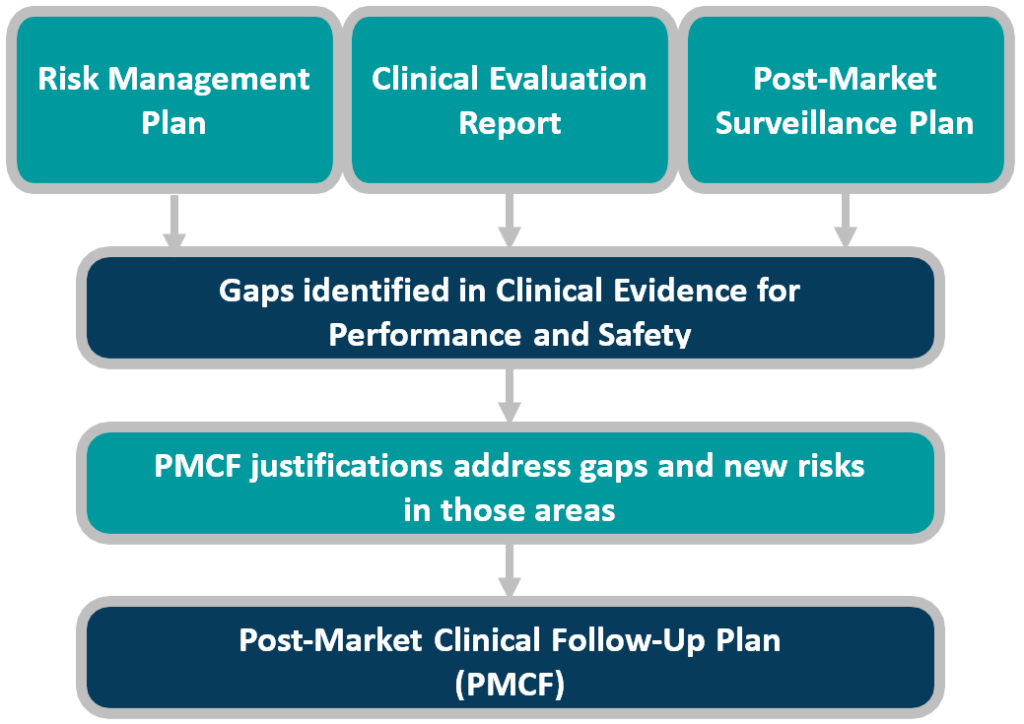
Does Your Organization's Post-Market Clinical Follow-Up (PMCF) Plan Adequately Reflect the Intensity Required in the Clinical Evaluation Report (CER) Under the Newest Medical Device Regulations? - Criterion Edge